Last week, in
Fusion Fuels: Part 1 - The isotopes of hydrogen, I described the three isotopes of hydrogen and why we might need them as fuel when we finally develop a fusion reactor on earth. If deuterium and tritium are foreseen to be the fuels for fusion, where do we get deuterium?
Deuterium exists in all the water that you ever see anywhere. On average in Standard Mean Ocean Water (SMOW) there is one deuterium atom for every 6400 hydrogen atoms, so although it is somewhat dilute it is not particularly rare. In some places on earth you can find water that is slightly enriched in deuterium - with perhaps double the concentration.
Browsing around the internet you can find claims that the Dead Sea is one of these areas. Lake Tangyanika is another. These claims are plausible. The one thing in common between these places is that they have rivers
and streams running into them, but no river running out of them. Water
only leaves them by evaporation and the vapour evaporating from a water surface tends to be richer in the light isotope, protium, than in the heavier deuterium. Therefore the water left behind in closed bodies of water is likely to be slightly enriched in deuterium. The water might reach double the concentration of normal water, but no better than that.
You might also find claims that the water in the deepest ocean trenches is enriched in deuterium. One could propose that this is because the 'heavy water' sinks to the bottom of the ocean and collects there. For now I am going to resist the temptation to address this topic very much, because I am very skeptical about it. In fact the only evidence that I have ever found about the deuterium concentration in deep ocean water suggests that the claim is unfounded.
In order to obtain pure deuterium, water has to be processed in a variety of ways. There is no single process that produces pure deuterium from ordinary water in one step. In fact it is usually achieved by three distinct and sequential processes. The first is designed to concentrate the deuterium to between 20 and 30% in water and the others to increase the concentration further and to generate deuterium gas from this 'heavy water'.
The initial stages are usually achieved by a technique known as 'isotopic exchange'. The three different hydrogen isotopes very easily swap places with each other in molecules. Hydrogen, deuterium and tritium are chemically and physically equivalent in most situations, and a water molecule containing two hydrogen atoms and one oxygen atom can easily become one that contains a hydrogen, a deuterium and an oxygen. The forms of water that can be produced in the presence of H and D are H
2O, HDO, D
2O. If tritium is included in the equation, three other forms can be produced, specifically HTO, DTO, T
2O. Tritium doesn't exist for long in nature so let's ignore it for now.
So it is
not very inaccurate to say that all the water you have ever seen or drunk, or washed or swum in was almost all H
2O, but that one molecule in 6400 was HDO. There is so little D
2O in nature that we need not worry at all about the health hazards associated with drinking pure heavy water.
If we want to increase the concentration of D in the water and to make significant amounts of D
2O, we have to 'trick it', and one way to do that is to use another molecule containing hydrogen. Hydrogen sulphide (H
2S) and ammonia (NH
3) are both commonly used but for simplicity I will just describe the use of the former of these two.
Less than 100 years ago, two people spotted that if you bubble hydrogen sulphide through hot water (say 130 degrees celsius, at high pressure to prevent it boiling), the deuterium can be made to jump from the HDO molecules into the H
2S to make HDS. They also noticed that in colder water (say 30 degrees celsius) the deuterium tends to jump the other way to make HDO or D
2O. They carefully explored how this property changed as they varied the temperature and the end result was the Geib-Spevack (GS) process. The process was industrialised by a North American company called Girdler, and no doubt there was ill feeling about admitting that the inventors were German at the time, because the company quietly made sure that it mutated the apparent meaning of 'GS' to '
Girlder Sulfide'. In the English-speaking parts of the world, that name has prevailed.
(Although I know a lot of Germans, none of them have admitted to me that they know about industrial processes to separate deuterium so I can't confirm what they call it.)
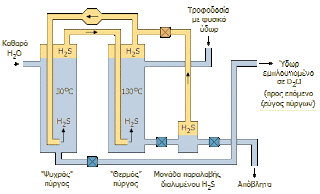 |
GS process (annotated in Greek) but still useful
to an English speaking audience (from here) |
By circulating the hydrogen sulfide through hot and cold water continuously, you can deplete the deuterium from the hot water and concentrate it in the cold water. This sounds easy enough, and you might think it could be engineered properly to ensure that it doesn't take a huge amount of energy, but there are a few practical difficulties. For a start, hydrogen sulphide is toxic, so you have to be very careful with it. (Ammonia is not much better by the way.) Secondly, the physics only works for you until the concentration of deuterium reaches about 30% (limit), and for most practical purposes not much more than 20%. After that you can wait as long as you like, but you won't improve it further.
Still - looking at it positively, you have concentrated the deuterium from 155 parts per million to more that one part in five. That's not a bad start!
To go further than this, you have to change tactics and use a process known as
vacuum distillation to do what nature does in the lakes I mentioned earlier - but to do it better.
More next time.
Fusion Fuels part 3 - Making 'heavy water'
Other articles in this series:
Fusion Fuels: Part 1 - The isotopes of hydrogen
Fusion fuels 4 - Electrolysis of heavy water
Fusion Fuels part 5 - Tritium












